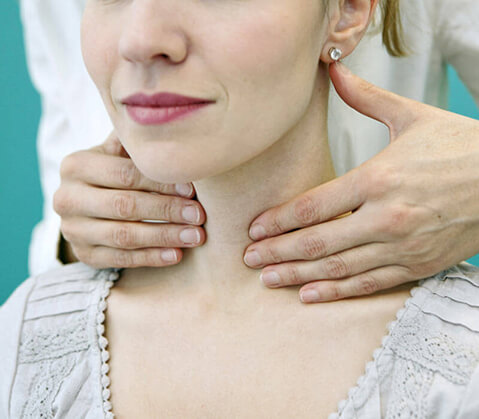
Chicago ENT is currently enrolling patients in a clinical study of early-onset tinnitus diagnosed within one ear.
This study is researching an investigational new drug to see if it might help people with tinnitus. Investigational means it has not been approved by any regulatory agency and can only be used in clinical research studies. This study will help researchers learn more about tinnitus and the study drug.
The study drug is given as an injection in the middle ear. To receive the injection, a trained medical professional will numb a part of your ear drum, insert a small needle into your middle ear, and inject the study drug.
Qualified participants will receive at no cost:
- Study-related lab tests, questionnaires, and physical exams
- Possible access to the study drug (some participants will receive placebo, which has no active ingredients)
Education about tinnitus
- If you are interested, you will receive tests and assessments to make sure you meet all the criteria to participate. If you qualify, your participation will last about 16-20 weeks.
To receive more information about this study, or if you or someone you know may qualify, contact:
Ninos Joseph at 773-289-1823 or [email protected]