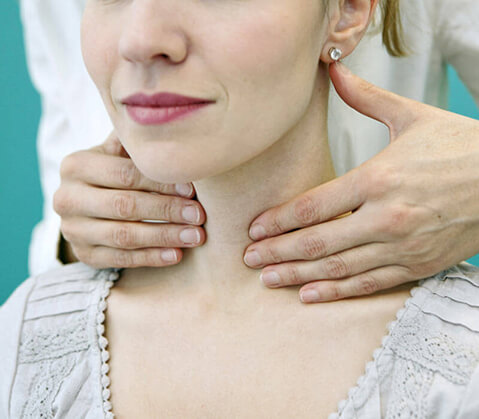
FDA announces voluntary recall of Montelukast tablets by Camber Pharmaceuticals
Note that this is for 10 mg tablets in a 30-day supply pill bottle. The lot number for the recalled product is MON17384, the expiration date is 12/31/2019, and the national drug code is 31722-726-30. You can read the full release from the FDA at this link.
Managing Asthma in Urban Areas
Dr. Payal Patel is Part of Expert Panel Asthma is a major noncommunicable disease prevalent across the United States in 7.6% of adults and 8.4% of children. In Chicago, 10.8% of adults reported having asthma in 2014. In Illinois, the percentage of adults and youth reporting that they are currently having asthma is higher than… Read More